Copper(I) thiocyanate
 Copper(I) thiocyanate
| |
| |
| Names | |
|---|---|
| Other names
Cuprous thiocyanate
| |
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | 100.012.894 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| CuSCN | |
| Molar mass | 121.628 g/mol[1] |
| Appearance | white powder |
| Density | 2.88 g/cm3[2] |
| Melting point | 1,084[1] °C (1,983 °F; 1,357 K) |
| 8.427·10−7 g/L (20 °C) | |
Solubility product (Ksp)
|
1.77×10−13[3] |
| -48.0·10−6 cm3/mol | |
| Related compounds | |
Other anions
|
Copper(I) iodide, copper(I) cyanide |
Other cations
|
Ammonium thiocyanate Potassium thiocyanate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Copper(I) thiocyanate (or cuprous thiocyanate) is a coordination polymer with formula CuSCN. It is an air-stable, white solid used as a precursor for the preparation of other thiocyanate salts.
Structure
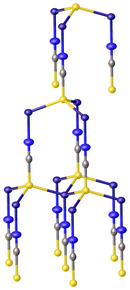
[edit]At least two polymorphs have been characterized by X-ray crystallography. They both feature copper(I) in a characteristic tetrahedral coordination geometry. The sulfur end of the SCN- ligand is triply bridging so that the coordination sphere for copper is CuS3N.[2][4]
Synthesis
[edit]Copper(I) thiocyanate forms from the spontaneous decomposition of black copper(II) thiocyanate, releasing thiocyanogen, especially when heated.[5] It is also formed from copper(II) thiocyanate under water, releasing (among others) thiocyanic acid and the highly poisonous hydrogen cyanide.[6] It is conveniently prepared from relatively dilute solutions of copper(II) in water, such as copper(II) sulphate. To a copper(II) solution sulphurous acid is added and then a soluble thiocyanate is added (preferably slowly, while stirring[7]). Copper(I) thiocyanate is precipitated as a white powder.[8] Alternatively, a thiosulfate solution may be used as a reducing agent.
Double salts
[edit]Copper(I) thiocyanate forms one double salt with the group 1 elements, CsCu(SCN)2. The double salt only forms from concentrated solutions of CsSCN, into which CuSCN dissolves. From less concentrated solutions, solid CuSCN separates reflecting its low solubility.[9] When brought together with potassium, sodium or barium thiocyanate, and brought to crystallisation by concentrating the solution, mixed salts will crystallise out. These are not considered true double salts. As with CsCu (SNC)2, copper(I) thiocyanate separates out when these mixed salts are redissolved or their solutions diluted.[10]
Uses
[edit]Copper(I) thiocyanate is a hole conductor, a semiconductor with a wide band gap (3.6 eV, therefore transparent to visible and near infrared light).[11] It is used in photovoltaics in some third-generation cells as a hole transfer layer. It acts as a P-type semiconductor and as a solid-state electrolyte. It is often used in dye-sensitized solar cells. Its hole conductivity is however relatively poor (0.01 S·m−1). This can be improved by various treatments, e.g. exposure to gaseous chlorine or doping with (SCN)2.[12]
CuSCN with NiO act synergically as a smoke suppressant additive in polyvinyl chloride (PVC).
CuSCN precipitated on carbon support can be used for conversion of aryl halides to aryl thiocyanates.[13]
Copper thiocyanate is used in some anti-fouling paints.[14][15] Advantages compared to cuprous oxide include that the compound is white and a more efficient biocide.
References
[edit]- ^ a b "Properties of Copper(I) thiocyanate". Chemspider. Alfa Aesar 40220. Retrieved 5 January 2016.
- ^ a b Smith, D. L.; Saunders, V. I. "Preparation and Structure Refinement of the 2H Polytype of beta-Copper(I) Thiocyanate" Acta Crystallographica B, 1982, volume 38, 907-909. doi:10.1107/S0567740882004361
- ^ John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- ^ Kabešová, M.; Dunaj-Jurčo, M.; Serator, M.; Gažo, J.; Garaj, J. (1976). "The Crystal Structure of Copper(I) Thiocyanate and Its Relation to the Crystal Structure of Copper(II) Diammine Dithiocyanate Complex". Inorganica Chimica Acta. 17: 161–165. doi:10.1016/S0020-1693(00)81976-3.
- ^ Hunter, J. A.; Massie, W. H. S.; Meiklejohn, J.; Reid, J. (1969-01-01). "Thermal rearrangement in copper(II) thiocyanate". Inorganic and Nuclear Chemistry Letters. 5 (1): 1–4. doi:10.1016/0020-1650(69)80226-6. ISSN 0020-1650.
- ^ David Tudela (1993). "The Reaction of Copper(II) with Thiocyanate Ions (Letter to the Editor)". Journal of Chemical Education. 70 (2): 174. doi:10.1021/ed070p174.3.PDF copy
- ^ Matthew Dick (1969). "Use of cuprous thiocyanate as a short-term continuous marker for faeces". Gut. 10 (5): 408–412 (408). doi:10.1136/gut.10.5.408. PMC 1552857. PMID 5771673.PDF copy
- ^ Reece H. Vallance, Douglas F. Twiss and Miss Annie R. Russell (1931). J. Newton Friend (ed.). A text-book of inorganic chemistry, volume VII, part II. Charles Griffin & Company Ltd. p. 282.
- ^ H.L.Wells (1902). "On some double and triple thiocyanates". American Chemical Journal. 28: 245–284 (263).
- ^ Herbert E. Williams (1915). The chemistry of cyanogen compounds. J. & A. Churchill, London. pp. 202–203.
- ^ Wilde, G. (2009). Nanostructured Materials. Elsevier Science. p. 256. ISBN 9780080914237. Retrieved 14 January 2017.
- ^ Albini, A.; Fausto, R.; de Melo, J.S.S.; Maldotti, A.; Clementi, C.; Kalyanasundaram, K.; Johnston, L.J.; Harbron, E.; Misawa, H.; Romani, A. (2011). Photochemistry. Royal Society of Chemistry. p. 164. ISBN 9781849731652. Retrieved 14 January 2017.
- ^ Clark, J.H.; Kybett, A.P.; Macquarrie, D.J. (1992). Supported Reagents: Preparation, Analysis, and Applications. Wiley. p. 121. ISBN 9780471187790. Retrieved 14 January 2017.
- ^ "Copper in Antifouling". Archived from the original on 2017-04-27. Retrieved 2017-04-25.
- ^ Vetere, V. F.; Pérez, M. C.; Romagnoli, R.; Stupak, M. E.; Amo, B. (1997). "Solubility and toxic effect of the cuprous thiocyanate antifouling pigment on barnacle larvae". Journal of Coatings Technology. 69 (3): 39–45. doi:10.1007/BF02696144.
